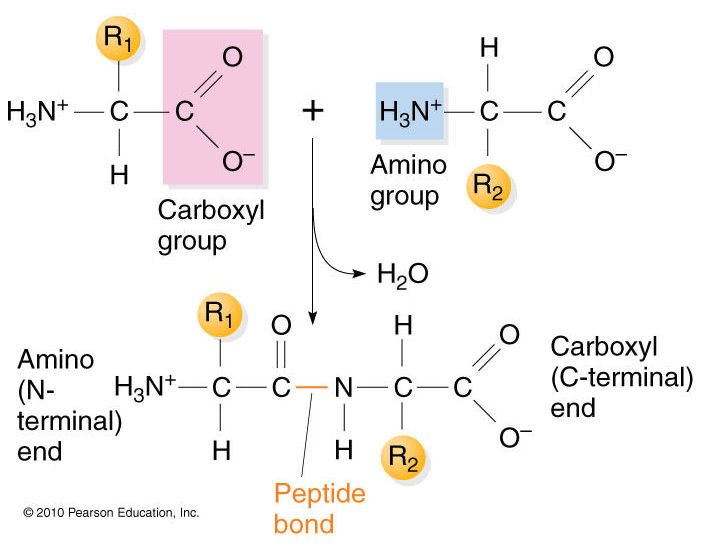
Are Amino Acids Linked By Hydrogen Bonds . Hydrophobic side chains interact with each other via weak van. Charged amino acid side chains can form ionic bonds, and polar amino acids are capable of forming hydrogen bonds. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (h +) ion, and the amino acid. Amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next,. These amino acids are joined by peptide bonds from the carboxyl terminal of one amino acid to the amino terminal of the next. Proteins are one of the most important classes of molecules for life and underpin the field of biochemistry. Secondary structure refers to the localized, simple, shapes that.
from sphweb.bumc.bu.edu
Proteins are one of the most important classes of molecules for life and underpin the field of biochemistry. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (h +) ion, and the amino acid. Amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next,. These amino acids are joined by peptide bonds from the carboxyl terminal of one amino acid to the amino terminal of the next. Hydrophobic side chains interact with each other via weak van. Secondary structure refers to the localized, simple, shapes that. Charged amino acid side chains can form ionic bonds, and polar amino acids are capable of forming hydrogen bonds.
Nucleic Acids
Are Amino Acids Linked By Hydrogen Bonds Charged amino acid side chains can form ionic bonds, and polar amino acids are capable of forming hydrogen bonds. Secondary structure refers to the localized, simple, shapes that. Hydrophobic side chains interact with each other via weak van. Charged amino acid side chains can form ionic bonds, and polar amino acids are capable of forming hydrogen bonds. These amino acids are joined by peptide bonds from the carboxyl terminal of one amino acid to the amino terminal of the next. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (h +) ion, and the amino acid. Amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next,. Proteins are one of the most important classes of molecules for life and underpin the field of biochemistry.
From www.slideserve.com
PPT Special Topics PowerPoint Presentation, free download ID4808084 Are Amino Acids Linked By Hydrogen Bonds If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (h +) ion, and the amino acid. Hydrophobic side chains interact with each other via weak van. These amino acids are joined by peptide bonds from the carboxyl terminal of one amino acid to the amino terminal of the next. Secondary structure refers to. Are Amino Acids Linked By Hydrogen Bonds.
From www.youtube.com
How to calculate hydrogen bonds in AlphaHelix YouTube Are Amino Acids Linked By Hydrogen Bonds Hydrophobic side chains interact with each other via weak van. Amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next,. Secondary structure refers to the localized, simple, shapes that. If acid is added to a solution containing the zwitterion, the carboxylate group. Are Amino Acids Linked By Hydrogen Bonds.
From www.espacecorps-espritforme.fr
Protéine, Acide aminé, Peptide Définition simple et complète Espace Are Amino Acids Linked By Hydrogen Bonds Proteins are one of the most important classes of molecules for life and underpin the field of biochemistry. Hydrophobic side chains interact with each other via weak van. Amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next,. Charged amino acid side. Are Amino Acids Linked By Hydrogen Bonds.
From saylordotorg.github.io
Amino Acids, Proteins, and Enzymes Are Amino Acids Linked By Hydrogen Bonds Proteins are one of the most important classes of molecules for life and underpin the field of biochemistry. Hydrophobic side chains interact with each other via weak van. Amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next,. Secondary structure refers to. Are Amino Acids Linked By Hydrogen Bonds.
From www.expii.com
Amino Acids & Polypeptide Chains — Structure & Synthesis Expii Are Amino Acids Linked By Hydrogen Bonds Secondary structure refers to the localized, simple, shapes that. These amino acids are joined by peptide bonds from the carboxyl terminal of one amino acid to the amino terminal of the next. Hydrophobic side chains interact with each other via weak van. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (h +). Are Amino Acids Linked By Hydrogen Bonds.
From sphweb.bumc.bu.edu
Nucleic Acids Are Amino Acids Linked By Hydrogen Bonds Amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next,. Secondary structure refers to the localized, simple, shapes that. Charged amino acid side chains can form ionic bonds, and polar amino acids are capable of forming hydrogen bonds. Hydrophobic side chains interact. Are Amino Acids Linked By Hydrogen Bonds.
From www.researchgate.net
(a) Amino acid structure. (b) Peptide bond formation. (c) Schematic of Are Amino Acids Linked By Hydrogen Bonds Charged amino acid side chains can form ionic bonds, and polar amino acids are capable of forming hydrogen bonds. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (h +) ion, and the amino acid. Proteins are one of the most important classes of molecules for life and underpin the field of biochemistry.. Are Amino Acids Linked By Hydrogen Bonds.
From www.coursehero.com
Amino Acids Structure Nutrition Course Hero Are Amino Acids Linked By Hydrogen Bonds Hydrophobic side chains interact with each other via weak van. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (h +) ion, and the amino acid. Amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the. Are Amino Acids Linked By Hydrogen Bonds.
From donate-faqs.com
Which Amino Acids Can Donate A Hydrogen Bond Are Amino Acids Linked By Hydrogen Bonds Hydrophobic side chains interact with each other via weak van. Amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next,. These amino acids are joined by peptide bonds from the carboxyl terminal of one amino acid to the amino terminal of the. Are Amino Acids Linked By Hydrogen Bonds.
From socratic.org
What type of interaction is depicted by the dashed line? Socratic Are Amino Acids Linked By Hydrogen Bonds Amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next,. Proteins are one of the most important classes of molecules for life and underpin the field of biochemistry. Hydrophobic side chains interact with each other via weak van. If acid is added. Are Amino Acids Linked By Hydrogen Bonds.
From biochemanics.wordpress.com
Amino Acids biochemanics Are Amino Acids Linked By Hydrogen Bonds Amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next,. Proteins are one of the most important classes of molecules for life and underpin the field of biochemistry. Secondary structure refers to the localized, simple, shapes that. Hydrophobic side chains interact with. Are Amino Acids Linked By Hydrogen Bonds.
From philschatz.com
Organic Compounds Essential to Human Functioning · Anatomy and Physiology Are Amino Acids Linked By Hydrogen Bonds Amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next,. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (h +) ion, and the amino acid. Charged amino acid side chains can form ionic bonds,. Are Amino Acids Linked By Hydrogen Bonds.
From www.shutterstock.com
Transient Hydrogen Bonds Protein Formation Amino Stock Vector (Royalty Are Amino Acids Linked By Hydrogen Bonds These amino acids are joined by peptide bonds from the carboxyl terminal of one amino acid to the amino terminal of the next. Proteins are one of the most important classes of molecules for life and underpin the field of biochemistry. Secondary structure refers to the localized, simple, shapes that. If acid is added to a solution containing the zwitterion,. Are Amino Acids Linked By Hydrogen Bonds.
From philschatz.com
Amines and Amides · Chemistry Are Amino Acids Linked By Hydrogen Bonds Proteins are one of the most important classes of molecules for life and underpin the field of biochemistry. Secondary structure refers to the localized, simple, shapes that. These amino acids are joined by peptide bonds from the carboxyl terminal of one amino acid to the amino terminal of the next. If acid is added to a solution containing the zwitterion,. Are Amino Acids Linked By Hydrogen Bonds.
From ar.inspiredpencil.com
The Reaction Between Two Amino Acids Are Amino Acids Linked By Hydrogen Bonds These amino acids are joined by peptide bonds from the carboxyl terminal of one amino acid to the amino terminal of the next. Amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next,. Hydrophobic side chains interact with each other via weak. Are Amino Acids Linked By Hydrogen Bonds.
From opened.cuny.edu
Biology 2e, The Chemistry of Life, Biological Macromolecules, Proteins Are Amino Acids Linked By Hydrogen Bonds Proteins are one of the most important classes of molecules for life and underpin the field of biochemistry. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (h +) ion, and the amino acid. Amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid. Are Amino Acids Linked By Hydrogen Bonds.
From philschatz.com
Proteins · Microbiology Are Amino Acids Linked By Hydrogen Bonds Amino acids are linked to each other by peptide bonds, in which the carboxyl group of one amino acid is joined to the amino group of the next,. Secondary structure refers to the localized, simple, shapes that. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (h +) ion, and the amino acid.. Are Amino Acids Linked By Hydrogen Bonds.
From courses.lumenlearning.com
Reading Protein Structure Biology I Are Amino Acids Linked By Hydrogen Bonds Proteins are one of the most important classes of molecules for life and underpin the field of biochemistry. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (h +) ion, and the amino acid. These amino acids are joined by peptide bonds from the carboxyl terminal of one amino acid to the amino. Are Amino Acids Linked By Hydrogen Bonds.